Water Conditioning And How It Works
We think of water as a clear liquid but water really contains suspended solids as well as dissolved minerals and gases. These components are picked up as water passes through nature on the way to our homes. Water hardness is measured by the amount of dissolved calcium and magnesium in water.
Note: While magnesium contributes to the hardness level it’s calcium that causes hard scale, so that’s what we address here.
How Does Hard Water Cause Us Problems?
Water will only dissolve a certain amount of calcium dependent upon conditions. The amount of dissolved calcium that can be held in water reduces as temperature or pH increases. Using temperature as an example, let’s take a hard water and heat it to a temperature (x) where the water is at its absolute maximum capacity for dissolved calcium. This is known as the saturation point. Now we’ll continue to apply heat taking the temperature above (x). As we exceed the saturation point an amount of dissolved calcium is forced out of solution (it precipitates meaning it is no longer dissolved).
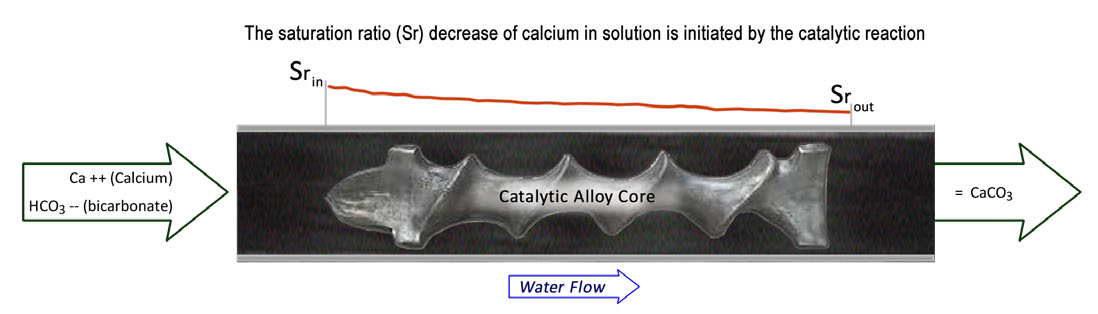
When calcium precipitates in this way it can combine with bicarbonate to form a hard calcium carbonate scale called calcite that bonds to the nearest receptive surface.
To prevent this from taking place our treatment reduces the amount of dissolved calcium by precipitating calcium carbonate in a form called aragonite, harmless insoluble crystals that are carried through the system to the drain or consumed.


Our Treatment Uses A Catalytic Reaction To Induce The Precipitation Of Aragonite (Details Below)
- The NaturalSof scale prevention product line utilizes a non-sacrificial lead free alloy core with a special surface. As water passes over the core a catalytic reaction takes place.
- The reaction causes carbonic acid to precipitate. The reduction of this acidic component increases the pH of the solution.
- This pH increase triggers calcium and bicarbonate to come out of solution combining to form calcium carbonate (CaCO3) in its aragonite state. The pH increase is only temporary so there’s no difference in pH readings before and after the unit.
- Catalytic treated water has a greater capacity for calcium. This greater capacity prevents scale deposition and in many cases any pre-existing scale is gradually absorbed.
Have Any Questions or Want a Free Estimate?
Contact the NaturalSof Dealer Network today, to find a certified contractor in your area. Fill out this easy form to see the difference clean, healthy water can make.
